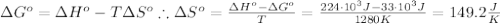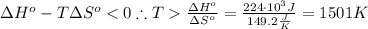The standard reaction enthalpy of zn(s) + h2o(g) →zno(s) + h2(g) is known to be hr 0 = 224 kj and is approximately constant from 920 k up to 1280 k. the standard reaction free energy is +33 kj at 1280 k. calculate the equilibrium constant at 1280 k and then calculate the temperature at which the equilibrium constant becomes greater than 1.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

You know the right answer?
The standard reaction enthalpy of zn(s) + h2o(g) →zno(s) + h2(g) is known to be hr 0 = 224 kj and i...
Questions


Advanced Placement (AP), 07.05.2021 06:00

English, 07.05.2021 06:00




Social Studies, 07.05.2021 06:00

History, 07.05.2021 06:00


Mathematics, 07.05.2021 06:00


Mathematics, 07.05.2021 06:00


Mathematics, 07.05.2021 06:00




Mathematics, 07.05.2021 06:00

Mathematics, 07.05.2021 06:00

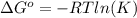
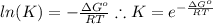
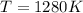 and the ideal gas law constant
and the ideal gas law constant 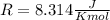 , we obtain:
, we obtain: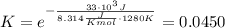
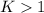 , then
, then 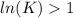 and
and 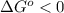 .
.