Using vsepr theory, which of these molecules does not have a trigonal planar shape?
a) ammoni...


Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

You know the right answer?
Questions








Computers and Technology, 24.02.2020 16:08


Computers and Technology, 24.02.2020 16:08







Spanish, 24.02.2020 16:09



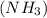 has 3 sigma bonds and one lone pair.Hence its shape is pyramidal due to bond pair - lone pair repulsion.Formaldehyde (HCHO) has 3 sigma bonds and 1 pi bond.Hence it has trigonal planar shape.Sulfur trioxide
has 3 sigma bonds and one lone pair.Hence its shape is pyramidal due to bond pair - lone pair repulsion.Formaldehyde (HCHO) has 3 sigma bonds and 1 pi bond.Hence it has trigonal planar shape.Sulfur trioxide 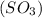 has 3 sigma bonds and 3 pi bonds.Boron triflouride (BF_3) has 3 sigma bonds.
has 3 sigma bonds and 3 pi bonds.Boron triflouride (BF_3) has 3 sigma bonds.

