
Chemistry, 17.12.2019 21:31 CassidgTab
Which of the following explains the vsepr geometry of a water molecule?
a. it is bent because there are four bonded pairs around oxygen.
b. it is tetrahedral because there are four bonded pairs around oxygen.
c. it is bend because there are two bonded pairs and two tons pairs around oxygen.
d. it is tetrahedral because there are two bonded pairs and two lone pairs around oxygen

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Which of the following explains the vsepr geometry of a water molecule?
a. it is bent becaus...
a. it is bent becaus...
Questions





History, 15.02.2021 07:20

Mathematics, 15.02.2021 07:20

English, 15.02.2021 07:20

Biology, 15.02.2021 07:20

Health, 15.02.2021 07:20

Social Studies, 15.02.2021 07:20

Mathematics, 15.02.2021 07:20

Mathematics, 15.02.2021 07:20

Geography, 15.02.2021 07:20



Mathematics, 15.02.2021 07:20



Social Studies, 15.02.2021 07:20

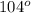 .
.

