
Hich one of the following statements about orbital hybridization is incorrect?
m
a. the carbon atom in ch4 is sp3 hybridized.
b. the carbon atom in co2 is sp hybridized.
c. the nitrogen atom in nh3 is sp2 hybridized.
d. sp2 hybrid orbitals are coplanar, and at 120 to each other.
e. sp hybrid orbitals lie at 180° to each other.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
Hich one of the following statements about orbital hybridization is incorrect?
m
a. the...
m
a. the...
Questions


History, 13.07.2019 06:50


Social Studies, 13.07.2019 06:50

Biology, 13.07.2019 06:50

Biology, 13.07.2019 06:50

Social Studies, 13.07.2019 06:50



Biology, 13.07.2019 06:50

Social Studies, 13.07.2019 06:50

Biology, 13.07.2019 06:50

Mathematics, 13.07.2019 06:50

History, 13.07.2019 06:50






![\text{Number of electron pair}=\frac{1}{2}[V+N-C+A]](/tpl/images/0422/7584/9e987.png)
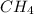
![\text{Number of electrons}=\frac{1}{2}\times [4+4]=4](/tpl/images/0422/7584/b4293.png)
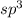 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.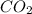
![\text{Number of electrons}=\frac{1}{2}\times [4]=2](/tpl/images/0422/7584/34bd3.png)
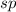 and the electronic geometry of the molecule will be linear.
and the electronic geometry of the molecule will be linear.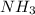
![\text{Number of electrons}=\frac{1}{2}\times [5+3]=4](/tpl/images/0422/7584/5f4d9.png)


