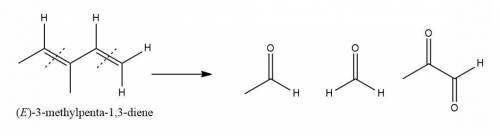
An unknown compound with empirical formula c3h5 was treated with br2/ccl4. the bromine solution went from orangish/red to clear immediately at room temperature. upon treatment with o3 followed by work-up with dimethylsulfide the following products were identified. from the information provided what is/are the most likely structure(s) for this unknown compound.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2


Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
An unknown compound with empirical formula c3h5 was treated with br2/ccl4. the bromine solution went...
Questions


Social Studies, 18.11.2020 23:00

Mathematics, 18.11.2020 23:00


English, 18.11.2020 23:00

English, 18.11.2020 23:00

Mathematics, 18.11.2020 23:00






Mathematics, 18.11.2020 23:00



Mathematics, 18.11.2020 23:00

Mathematics, 18.11.2020 23:00



 .
.