
Chemistry, 17.12.2019 09:31 queenpaige2015
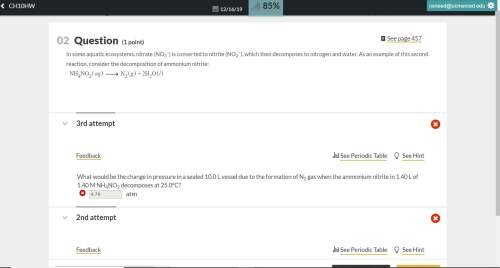
What would be the change in pressure in a sealed 10.0 l vessel due to the formation of n2 gas when the ammonium nitrite in 1.40 l of 1.40 m nh4no2 decomposes at 25.0°c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

You know the right answer?
What would be the change in pressure in a sealed 10.0 l vessel due to the formation of n2 gas when t...
Questions

Business, 31.08.2021 01:00






Social Studies, 31.08.2021 01:00




Mathematics, 31.08.2021 01:00

Mathematics, 31.08.2021 01:00

Mathematics, 31.08.2021 01:00

History, 31.08.2021 01:00

Mathematics, 31.08.2021 01:00



Mathematics, 31.08.2021 01:00

Biology, 31.08.2021 01:00

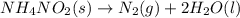
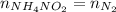
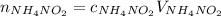
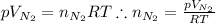
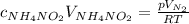
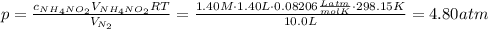
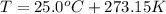 .
.

