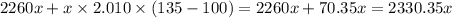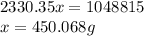Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
Consider a pot of water at 100 c. if it took 1,048,815 j of energy to vaporize the water and heat it...
Questions

History, 20.05.2021 14:00


English, 20.05.2021 14:00

Computers and Technology, 20.05.2021 14:00

History, 20.05.2021 14:00

Social Studies, 20.05.2021 14:00


History, 20.05.2021 14:00


Social Studies, 20.05.2021 14:00

Mathematics, 20.05.2021 14:00

Arts, 20.05.2021 14:00

English, 20.05.2021 14:00


Geography, 20.05.2021 14:00


Mathematics, 20.05.2021 14:00

Mathematics, 20.05.2021 14:00