
The reaction between c6h12o6 and o2 is represented by the balanced equation above. in an experiment, 0.30mol of co2 was produced from the reaction of 0.05mol of c6h12o6 with excess o2. the reaction was repeated at the same temperature and in the same container, but this time 0.60mol of co2 was produced. which of the following must be true

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 10:40
Question 17 hydrogen is manufactured on an industrial scale by this sequence of reactions: +ch4gh2og ⇌ +cog3h2g k1 +cogh2og ⇌ +co2gh2g k2 the net reaction is: +ch4g2h2og ⇌ +co2g4h2g k write an equation that gives the overall equilibrium constant k in terms of the equilibrium constants k1 and k2. if you need to include any physical constants, be sure you use their standard symbols, which you'll find in the aleks calculator.
Answers: 2

Chemistry, 23.06.2019 12:00
Which of the following statements is true? a. most heat energy is easily recovered and used for useful actions. b. friction causes molecules to vibrate more slowly. burning air and gasoline in an c. engine changes chemical energy into mechanical energy. it is impossible to d. change mechanical energy into mechanical energy.
Answers: 1
You know the right answer?
The reaction between c6h12o6 and o2 is represented by the balanced equation above. in an experiment,...
Questions










English, 02.08.2020 01:01










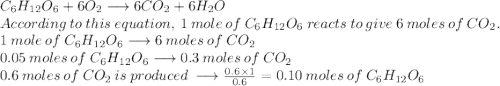
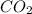 is produced when 0.1 mol of
is produced when 0.1 mol of 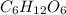 reacts with excess oxygen.
reacts with excess oxygen.

