
Nthe first step of the industrial process for making nitric acid, ammonia reacts with oxygen in the presence of a suitable catalyst to form nitric oxide and water vapor: 4 nh31g2+5 o21g2¡4 no1g2+6 h2o1g2how many liters of nh31g2 at 850 °c and 5.00 atm are required to react with 1.00 mol of o21g2 in this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

You know the right answer?
Nthe first step of the industrial process for making nitric acid, ammonia reacts with oxygen in the...
Questions


History, 22.08.2019 15:50

Mathematics, 22.08.2019 15:50


Mathematics, 22.08.2019 15:50

Chemistry, 22.08.2019 15:50

History, 22.08.2019 15:50






Mathematics, 22.08.2019 15:50

Biology, 22.08.2019 15:50

Mathematics, 22.08.2019 15:50

History, 22.08.2019 15:50


English, 22.08.2019 15:50

History, 22.08.2019 15:50

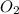 = 1.00 moles
= 1.00 moles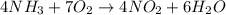
 moles of ammonia
moles of ammonia 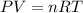
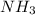 required.
required.


