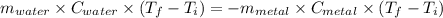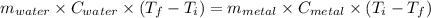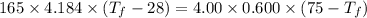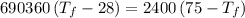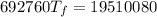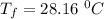Chemistry, 17.12.2019 04:31 aaronjcerrato
A4.00 g sample of a metal (specific heat = 0.600 j g-1°c-1 is heated to 75 degrees celcius and then dropped into 165 g of water in a calorimeter. what is the final temperature of the water if the initial temperature is 28 degrees celcius? the specific heat capacity of water is 4.184 j/g.°c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
A4.00 g sample of a metal (specific heat = 0.600 j g-1°c-1 is heated to 75 degrees celcius and then...
Questions


Social Studies, 06.11.2020 14:10

Computers and Technology, 06.11.2020 14:10

English, 06.11.2020 14:10



Chemistry, 06.11.2020 14:10


Chemistry, 06.11.2020 14:10


Mathematics, 06.11.2020 14:10


Mathematics, 06.11.2020 14:10

Mathematics, 06.11.2020 14:10


Mathematics, 06.11.2020 14:10

Advanced Placement (AP), 06.11.2020 14:10

Health, 06.11.2020 14:10

Engineering, 06.11.2020 14:10

Biology, 06.11.2020 14:10