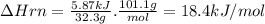Potassium nitrate, kno3, has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 32.3 g of kno3 is dissolved in 243 g of water at 23.00 °c. kno3(s)+h2o(aq) > koh(aq)+hno3(aq)the temperature of the resulting solution decreases to 17.90 °c. assume the resulting solution has the same specific heat as water, 4.184 j/(g·°c), and that there is negligible heat loss to the surroundings.1. how much heat was released by the solution? 2. what is the enthalpy of the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
Potassium nitrate, kno3, has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 32.3 g...
Questions

Mathematics, 13.10.2020 05:01


World Languages, 13.10.2020 05:01



Chemistry, 13.10.2020 05:01


World Languages, 13.10.2020 05:01




Chemistry, 13.10.2020 05:01





Mathematics, 13.10.2020 05:01

English, 13.10.2020 05:01


Mathematics, 13.10.2020 05:01