

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
Express the equilibrium constant for the following reaction.2 k(s) + 2 h2o(l) ↔ 2 koh(aq) + h2(g)k =...
Questions

English, 10.12.2021 23:10

Mathematics, 10.12.2021 23:10



Biology, 10.12.2021 23:10


Advanced Placement (AP), 10.12.2021 23:10

Mathematics, 10.12.2021 23:10

Mathematics, 10.12.2021 23:10


History, 10.12.2021 23:10




Advanced Placement (AP), 10.12.2021 23:10


Mathematics, 10.12.2021 23:10

Mathematics, 10.12.2021 23:10

World Languages, 10.12.2021 23:10

Mathematics, 10.12.2021 23:10

![K=[KOH]^2[H_2]](/tpl/images/0421/6907/b9417.png)
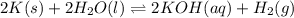
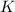 will be,
will be,

