

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1

Chemistry, 23.06.2019 14:00
How much would you need to weigh out in order to have 0.2moles of magnesium atoms?
Answers: 1
You know the right answer?
For each of the following pairs of substances, determine which has the larger molar entropy at 298 k...
Questions

Mathematics, 25.02.2021 05:30

English, 25.02.2021 05:30


Mathematics, 25.02.2021 05:30


Mathematics, 25.02.2021 05:30

Chemistry, 25.02.2021 05:30

Mathematics, 25.02.2021 05:30

History, 25.02.2021 05:30

Mathematics, 25.02.2021 05:30

Mathematics, 25.02.2021 05:30

English, 25.02.2021 05:30

Mathematics, 25.02.2021 05:30






Mathematics, 25.02.2021 05:30

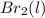 or
or 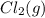 . Since, molar entropy of liquids is less than the molar entropy of gases.
. Since, molar entropy of liquids is less than the molar entropy of gases. 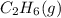 or
or 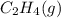 . As both the given species are gaseous in nature. So, more is the molar mass of specie more will be its molar entropy.
. As both the given species are gaseous in nature. So, more is the molar mass of specie more will be its molar entropy.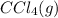 or
or 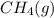 . Molar mass of
. Molar mass of 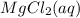 . Molar mass of NaCl 58.44 g/mol and molar mass of
. Molar mass of NaCl 58.44 g/mol and molar mass of 


