
Consider the following reaction at equilibrium. what effect will increasing the temperature have on the system? fe3o4(s) + co(g) ↔ 3 feo(s) + co2(g) δh°= +35.9 kjthe equilibrium constant will decrease. no effect will be observed. the reaction will shift to the right in the direction of products. the equilibrium constant will increase. the reaction will shift to the left in the direction of reactants

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
As a part of an experiment a student burns propane to produce carbon dioxide and water she remembers that she must follow the law conservation of matter when writing a balanced chemical equation which of these equation adheres to the law of conservation of matter
Answers: 1

Chemistry, 21.06.2019 19:00
The skeletal system performs a variety of functions that are crucial to maintaining life processes. what function is performed in the bone marrow, but not in the ossified bones of the skeleton? a oxygen transportation c mineral storage b. muscle attachment d red blood cell production
Answers: 3

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3
You know the right answer?
Consider the following reaction at equilibrium. what effect will increasing the temperature have on...
Questions


Business, 19.09.2019 21:30


Chemistry, 19.09.2019 21:30

Health, 19.09.2019 21:30


Spanish, 19.09.2019 21:30



History, 19.09.2019 21:30

Mathematics, 19.09.2019 21:30

Mathematics, 19.09.2019 21:30



Mathematics, 19.09.2019 21:30

History, 19.09.2019 21:30

Social Studies, 19.09.2019 21:30

English, 19.09.2019 21:30

History, 19.09.2019 21:30

Biology, 19.09.2019 21:30

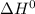 of the given reaction is positive and thus, on increasing temperature, reaction will go in forward direction.
of the given reaction is positive and thus, on increasing temperature, reaction will go in forward direction.

