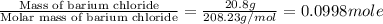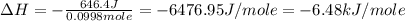Chemistry, 17.12.2019 00:31 raulhill98
An amount of solid barium chloride, 20.8 g, is dissolved in 100 g water in a coffee-cup calorimeter by the reaction: bacl2 (s) ba2+(aq) + 2cl−(aq) the water is originally at 25.0 °c and after the reaction the temperature of the solution is 26.6 °c. (cs = 4.04 j/(g°c) for the solution). what is the enthalpy change (δh) associated with the reaction as written?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

You know the right answer?
An amount of solid barium chloride, 20.8 g, is dissolved in 100 g water in a coffee-cup calorimeter...
Questions

Mathematics, 01.02.2020 11:42



Computers and Technology, 01.02.2020 11:42




Mathematics, 01.02.2020 11:43

Chemistry, 01.02.2020 11:43

Chemistry, 01.02.2020 11:43

Chemistry, 01.02.2020 11:43


Chemistry, 01.02.2020 11:43


History, 01.02.2020 11:43

World Languages, 01.02.2020 11:43


Mathematics, 01.02.2020 11:43


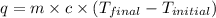
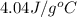
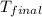 = final temperature =
= final temperature = 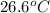
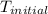 = initial temperature =
= initial temperature = 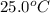
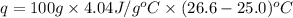
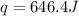
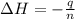
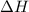 = enthalpy change = ?
= enthalpy change = ?