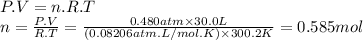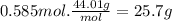Chemistry, 16.12.2019 22:31 cadenhuggins2
Carbon dioxide gas is collected at in an evacuated flask with a measured volume of . when all the gas has been collected, the pressure in the flask is measured to be . calculate the mass and number of moles of carbon dioxide gas that were collected. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3
You know the right answer?
Carbon dioxide gas is collected at in an evacuated flask with a measured volume of . when all the ga...
Questions

Mathematics, 14.10.2019 11:50






Mathematics, 14.10.2019 11:50


Biology, 14.10.2019 11:50

Mathematics, 14.10.2019 11:50



Mathematics, 14.10.2019 11:50






Mathematics, 14.10.2019 11:50