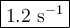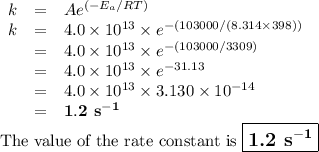Chemistry, 16.12.2019 22:31 caveman171
If the activation energy for a given compound is found to be 103 kj/mol, with a frequency factor of 4.0 × 1013 s-1, what is the rate constant for this reaction at 398 k? 2.5 × 107 s-18.2 s-13.9 × 1010 s-11.2 s-11.7 × 1010 s-1

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
What is the specific heat of a substance that absorbs 2.5×10^3 joules of heat when a sample of 1.0 ×10^4g of the substance increases in temperature from 10°c to 70°c?
Answers: 2

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1
You know the right answer?
If the activation energy for a given compound is found to be 103 kj/mol, with a frequency factor of...
Questions





Mathematics, 14.01.2021 17:50



Biology, 14.01.2021 17:50


History, 14.01.2021 17:50

Physics, 14.01.2021 17:50


Biology, 14.01.2021 17:50





English, 14.01.2021 17:50


English, 14.01.2021 17:50