
Chemistry, 16.12.2019 21:31 cschellfamily
Each of the following sets of quantum numbers is supposed to specify an orbital. choose the one set of quantum numbers that does not contain an error. each of the following sets of quantum numbers is supposed to specify an orbital. choose the one set of quantum numbers that does not contain an error. n = 4, l = 4, ml =0 n = 4, l = 0, ml =+1 n = 5, l = 3, ml =-3 n = 3, l = 1, ml = +2 n = 3, l = 2, ml =-3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Each of the following sets of quantum numbers is supposed to specify an orbital. choose the one set...
Questions

Chemistry, 04.02.2020 04:55

Mathematics, 04.02.2020 04:55

English, 04.02.2020 04:55

Mathematics, 04.02.2020 04:55

Biology, 04.02.2020 04:55




Mathematics, 04.02.2020 04:55

History, 04.02.2020 04:55




Spanish, 04.02.2020 04:55

Physics, 04.02.2020 04:55


Chemistry, 04.02.2020 04:55

Mathematics, 04.02.2020 04:55

History, 04.02.2020 04:55

Health, 04.02.2020 04:55

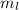 . The value of this quantum number ranges from
. The value of this quantum number ranges from 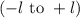 . When l = 2, the value of
. When l = 2, the value of 

