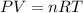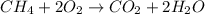Asample of methane gas having a volume of 2.80 l at 25 degree c and 1.65 atm was mixed with a sample of oxygen gas having a volume of 35.0 l at 31 degree c and 1.25 atm. the mixture was then ignited to form carbon dioxide and water. calculate the volume of co_2 formed at a pressure of 2.50 atm and a temperature of 125 degree c.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Asample of methane gas having a volume of 2.80 l at 25 degree c and 1.65 atm was mixed with a sample...
Questions



Advanced Placement (AP), 17.12.2020 21:50





Mathematics, 17.12.2020 21:50


Mathematics, 17.12.2020 21:50


History, 17.12.2020 21:50


Mathematics, 17.12.2020 21:50






Mathematics, 17.12.2020 21:50