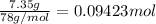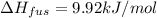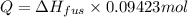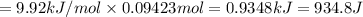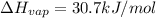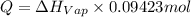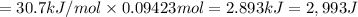Calculate the heat required to melt 7.35 g of benzene at its normal melting point. heat of fusion (benzene) = 9.92 kj/mol heat = kj
calculate the heat required to vaporize 7.35 g of benzene at its normal boiling point. heat of vaporization (benzene) = 30.7 kj/mol heat = kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Calculate the heat required to melt 7.35 g of benzene at its normal melting point. heat of fusion (b...
Questions











Chemistry, 19.09.2019 22:10









History, 19.09.2019 22:10