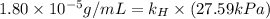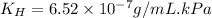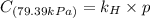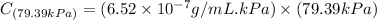Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 23.06.2019 10:20
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2

Chemistry, 23.06.2019 12:50
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1

Chemistry, 23.06.2019 16:30
All chemical reactions use reactants in a specific proportion or stoichiometry to form products. the reactant regulates the amount of products produced. a) excess b) limiting c) proportional d) stoichiometric
Answers: 1
You know the right answer?
Exposing a 250 ml sample of water at 20.∘c to an atmosphere containing a gaseous solute at 27.59 kpa...
Questions




Biology, 20.07.2019 12:30

Business, 20.07.2019 12:30

Biology, 20.07.2019 12:30

Biology, 20.07.2019 12:30






Mathematics, 20.07.2019 12:30

History, 20.07.2019 12:30






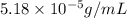
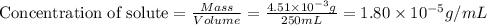
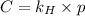
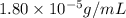
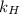 = Henry's law constant = ?
= Henry's law constant = ?