
Chemistry, 16.12.2019 17:31 samjohnson2383
Without doing any calculations, determine the sign of δssys for each of the following chemical reactions.2o3(g)→3o2(g)2h2s(g)+3o2( g)→2h2o(g)+2so2(g)so3(g)+h2o(l)→h2s o4(l)pcl3(l)+cl2(g)→pcl5(s)δssys greater than 0 or δssys smaller than 0

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
Without doing any calculations, determine the sign of δssys for each of the following chemical react...
Questions



Mathematics, 19.12.2020 06:40


Mathematics, 19.12.2020 06:40

Chemistry, 19.12.2020 06:40



Mathematics, 19.12.2020 06:40


Mathematics, 19.12.2020 06:40

Mathematics, 19.12.2020 06:40






Mathematics, 19.12.2020 06:40

Mathematics, 19.12.2020 06:40

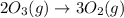 :
: 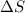 > 0
> 0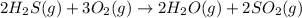 :
: 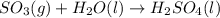 :
: 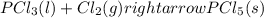 :
: 

