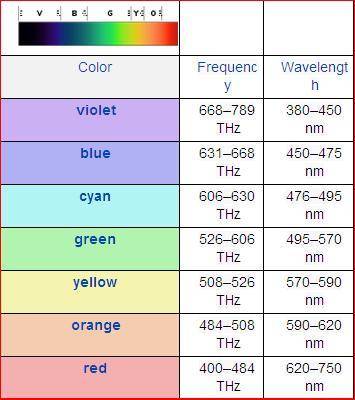
Chemistry, 14.12.2019 08:31 Nathaliasmiles
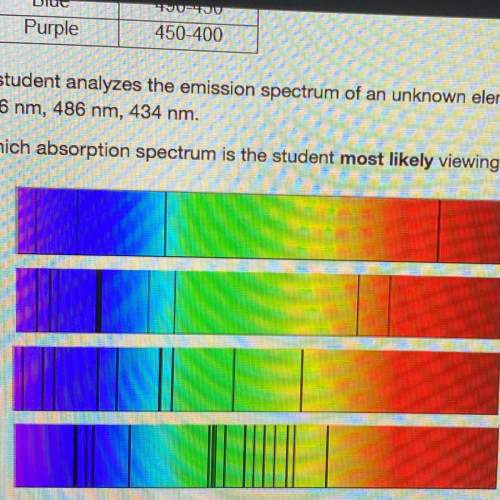
Astudent analyzes the emission spectrum of an unknown element and observes strong lines at the following wavelengths:
656 nm, 486 nm, 434 nm.
which absorption spectrum is the student most likely viewing?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

You know the right answer?
Astudent analyzes the emission spectrum of an unknown element and observes strong lines at the follo...
Questions

Mathematics, 19.05.2020 14:01

History, 19.05.2020 14:01


Health, 19.05.2020 14:01


Biology, 19.05.2020 14:01

Biology, 19.05.2020 14:01


Mathematics, 19.05.2020 14:01

Spanish, 19.05.2020 14:01






History, 19.05.2020 14:02


Mathematics, 19.05.2020 14:02

History, 19.05.2020 14:02

English, 19.05.2020 14:02