
Chemistry, 14.12.2019 06:31 braydenaddison738
Express the van der waals equation of state as a virial expansion in powers of 1/vm and obtain expressions for b and c in terms of the parameters a and b. the expansion you will need is (1 − x)−1 = 1 + x + x2 + … . measurements on argon gave b = −21.7 cm3 mol−1 and c = 1200 cm6 mol−2 for the virial coefficients at 273 k. what are the values of a and b in the corresponding van der waals equation of state?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1
You know the right answer?
Express the van der waals equation of state as a virial expansion in powers of 1/vm and obtain expre...
Questions

Mathematics, 23.11.2019 03:31

Mathematics, 23.11.2019 03:31

Mathematics, 23.11.2019 03:31




Business, 23.11.2019 03:31


Mathematics, 23.11.2019 03:31

Chemistry, 23.11.2019 03:31




History, 23.11.2019 03:31

Mathematics, 23.11.2019 03:31

Mathematics, 23.11.2019 03:31


Mathematics, 23.11.2019 03:31


![PV_{m} = RT[1 + (b-\frac{a}{RT})\frac{1}{V_{m} } + \frac{b^{2} }{V^{2} _{m} } + ...]](/tpl/images/0418/3833/bee7a.png)
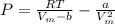
![P = RT[\frac{1}{V_{m}-b } - \frac{a}{RTV_{m} ^{2} }]](/tpl/images/0418/3833/a17f0.png)
![P = \frac{RT}{V_{m} } [\frac{1}{1-\frac{b}{V_{m} } } - \frac{a}{RTV_{m} }]](/tpl/images/0418/3833/173c2.png)
![PV_{m} = RT[(1-\frac{b}{V_{m} }) ^{-1} - \frac{a}{RTV_{m} }]](/tpl/images/0418/3833/0f37d.png)
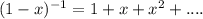
![PV_{m} = RT[1+\frac{b}{V_{m} }+\frac{b^{2} }{V_{m} ^{2} } + ... -\frac{a}{RTV_{m} }]](/tpl/images/0418/3833/a2cf6.png)
![P = RT[\frac{1}{V_{m} }+ \frac{B}{V_{m} ^{2}}+\frac{C}{V_{m} ^{3} }+ ...]](/tpl/images/0418/3833/ad91d.png)
![PV_{m} = RT[1+ \frac{B}{V_{m} }+ \frac{C}{V_{m} ^{2} } + ...]](/tpl/images/0418/3833/3ea1d.png) equation (2)
equation (2)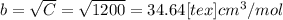 " alt="cm^{3}/mol" />" /> = 0.03464 L/mol
" alt="cm^{3}/mol" />" /> = 0.03464 L/mol


