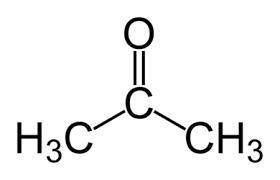
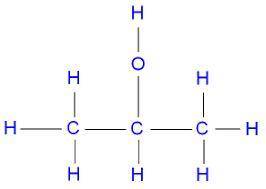
Identify the oxidation number of the highlighted carbon atoms in each of the molecules.
...

Chemistry, 14.12.2019 06:31 2023jpeterson
Identify the oxidation number of the highlighted carbon atoms in each of the molecules.
a. in molecule a a central carbon atom is bonded to two c h 3 groups and an o atom through a double bond.
b. the central carbon atom is highlighted.
c. in molecule b, a central carbon atom is bonded to two c h 3 groups, an o h group, and an h atom.
d. the central carbon atom is highlighted.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1


Chemistry, 23.06.2019 14:30
Which statement best identifies the process shown? the process must be fusion because energy is released. a.the process must be fusion because a heavier nucleus forms from smaller nuclei. b.the process must be fission because a large nucleus breaks into smaller nuclei. c.the process must be fission because neutrons are formed.
Answers: 1
You know the right answer?
Questions



History, 28.09.2020 19:01

History, 28.09.2020 19:01



English, 28.09.2020 19:01


Physics, 28.09.2020 19:01

Biology, 28.09.2020 19:01

Health, 28.09.2020 19:01




Physics, 28.09.2020 19:01

Mathematics, 28.09.2020 19:01



Biology, 28.09.2020 19:01

Mathematics, 28.09.2020 19:01

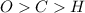
 always gets -1 and
always gets -1 and 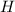 always gets +1 in these two compounds.The carbon - carbon bond will give 0 oxidation state.
always gets +1 in these two compounds.The carbon - carbon bond will give 0 oxidation state.