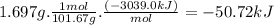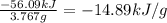Chemistry, 14.12.2019 03:31 mahmudabiazp3ekot
At constant volume, the heat of combustion of a particular compound, compound a, is − 3039.0 kj / mol. when 1.697 g of compound a (molar mass = 101.67 g / mol ) is burned in a bomb calorimeter, the temperature of the calorimeter (including its contents) rose by 3.661 ∘ c. what is the heat capacity (calorimeter constant) of the calorimeter? c = kj/°c suppose a 3.767 g sample of a second compound, compound b, is combusted in the same calorimeter, and the temperature rises from 23.23 ∘ c to 27.28 ∘ c. what is the heat of combustion per gram of compound b?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1


Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
You know the right answer?
At constant volume, the heat of combustion of a particular compound, compound a, is − 3039.0 kj / mo...
Questions





English, 07.09.2019 20:20




Chemistry, 07.09.2019 20:20


Advanced Placement (AP), 07.09.2019 20:20

Mathematics, 07.09.2019 20:20




Mathematics, 07.09.2019 20:20



Mathematics, 07.09.2019 20:20