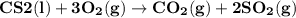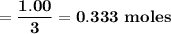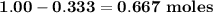Carbon disulfide burns in oxygen to yield carbon dioxide and sulfur dioxide according to the following chemical equation. cs2(l) + 3o2(g) → co2(g) + 2so2(g)
a. if 1.00 mol cs2 reacts with 1.00 mol o2, identify the limiting reactant.
b. how many moles of excess reactant remain?
c. how many moles of each product are formed?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

You know the right answer?
Carbon disulfide burns in oxygen to yield carbon dioxide and sulfur dioxide according to the followi...
Questions

Mathematics, 20.10.2020 22:01




Chemistry, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Physics, 20.10.2020 22:01

Chemistry, 20.10.2020 22:01


Social Studies, 20.10.2020 22:01



Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01


English, 20.10.2020 22:01


Mathematics, 20.10.2020 22:01