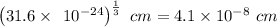The density of solid w is 19.3 g/cm^3. how many atoms are present per cubic centimeter of w?
...

Chemistry, 13.12.2019 23:31 erikamaldonado661
The density of solid w is 19.3 g/cm^3. how many atoms are present per cubic centimeter of w?
atoms cm^3
as a solid, w adopts a body-centered cubic unit cell. how many unit cells are present per cubic centimeter of w?
unit cells/cm^3
what is the volume of a unit cell of this metal?
cm^3
what is the edge length of a unit cell of w?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
Questions


Biology, 23.09.2019 06:30


Biology, 23.09.2019 06:30

Physics, 23.09.2019 06:30




Social Studies, 23.09.2019 06:30


Mathematics, 23.09.2019 06:30

Mathematics, 23.09.2019 06:30

Mathematics, 23.09.2019 06:30



Mathematics, 23.09.2019 06:30


Social Studies, 23.09.2019 06:30


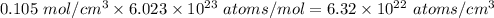
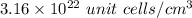
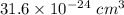
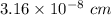
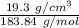 =0.105 mol/cm³
=0.105 mol/cm³
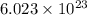 atoms.
atoms.
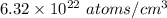
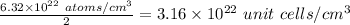
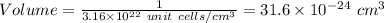
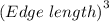
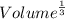 =
=