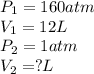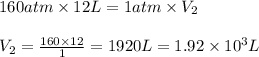According to boyle's law, for a fixed quantity of gas at a given temperature, what quantity relating pressure p and volume v is constant? view available hint(s) according to boyle's law, for a fixed quantity of gas at a given temperature, what quantity relating pressure and volume is constant?
a. pv
b. p×v
c. p+v
d. vp
application of boyle's law a 12-liter tank contains helium gas pressurized to 160 atm.
part b what size tank would be needed to contain this same amount of helium at atmospheric pressure (1 atm)? express the size in liters to three significant figures. view available hint(s) nothing ll

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 14:00
How much would you need to weigh out in order to have 0.2moles of magnesium atoms?
Answers: 1


Chemistry, 23.06.2019 22:30
How much heat (in kj) is evolved in converting 1.00 mol of steam at 130.0 ∘c to ice at -55.0 ∘c? the heat capacity of steam is 2.01 j/g⋅∘c and of ice is 2.09 j/g⋅∘c?
Answers: 1
You know the right answer?
According to boyle's law, for a fixed quantity of gas at a given temperature, what quantity relating...
Questions

English, 17.06.2021 17:10

Mathematics, 17.06.2021 17:10


Mathematics, 17.06.2021 17:10


English, 17.06.2021 17:10



Mathematics, 17.06.2021 17:10



Mathematics, 17.06.2021 17:10

English, 17.06.2021 17:10


Biology, 17.06.2021 17:10



Mathematics, 17.06.2021 17:10


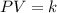 and the size of the tank must be
and the size of the tank must be 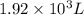
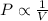
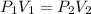
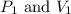 are initial pressure and volume.
are initial pressure and volume.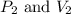 are final pressure and volume.
are final pressure and volume.