
For a first-order reaction, the half-life is constant. it depends only on the rate constant and not on the reactant concentration. it is expressed as: t1/2=0.693/k
for a second-order reaction, the half-life depends on the rate constant and the concentration of the reactant and so is expressed as: t1/2= 1/k[a]0
a. a certain first-order reaction (a--> products) has a rate constant of 3.30×10^-3 s^-1 at 45 degrees c. how many minutes does it take for the concentration of the reactant, [a], to drop to 6.25% of the original concentration?
b. a certain second-order reaction (b--> products) has a rate constant of 1.70×10^-3 m^-1*s^-1 at 27 degrees c and an initial half-life of 296 s. what is the concentration of the reactant b after one half-life?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
For a first-order reaction, the half-life is constant. it depends only on the rate constant and not...
Questions

Chemistry, 13.10.2019 22:30


Biology, 13.10.2019 22:30

English, 13.10.2019 22:30

World Languages, 13.10.2019 22:30


Social Studies, 13.10.2019 22:30


History, 13.10.2019 22:30




Mathematics, 13.10.2019 22:30


History, 13.10.2019 22:30





![[A_t]=[A_0]e^{-kt}](/tpl/images/0417/6687/1ef89.png)
![[A_t]](/tpl/images/0417/6687/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0417/6687/9a686.png) is the initial concentration
is the initial concentration
![\frac {[A_t]}{[A_0]}](/tpl/images/0417/6687/0d33c.png) = 0.0625
= 0.0625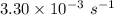
![\frac {[A_t]}{[A_0]}=e^{-k\times t}](/tpl/images/0417/6687/16cf4.png)
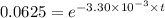
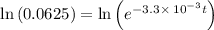
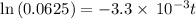
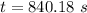
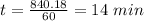
![t_{1/2}=\frac{1}{k[A_o]}](/tpl/images/0417/6687/4d220.png)
![[A_o]](/tpl/images/0417/6687/dc622.png) is the initial concentration = ?
is the initial concentration = ?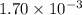 M⁻¹s⁻¹
M⁻¹s⁻¹
![296=\frac{1}{1.70\times 10^{-3}\times [A_o]}](/tpl/images/0417/6687/00cf0.png)
![296=\frac{1000}{1.7[A_o]}](/tpl/images/0417/6687/64d51.png)
![[A_o]=\frac{1250}{629}](/tpl/images/0417/6687/7cd99.png)
![[A_o]=1.99\ M](/tpl/images/0417/6687/59c0f.png)
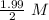 = 0.995 M
= 0.995 M

