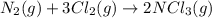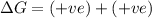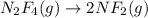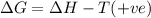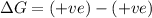Classify each of the following reactions as one of the four possible types:
1. spontan...

Chemistry, 13.12.2019 22:31 jose0765678755
Classify each of the following reactions as one of the four possible types:
1. spontaneous at all temperatures;
2. nonspontaneous at all temperatures;
3. spontaneous at low t; nonspontaneous at high t;
4. spontaneous at high t; nonspontaneous at low t.
(a) n2(g)+3f2(g)→2nf3(g); δh∘=−249kj; δs∘=−278j/k
(b) n2(g)+3cl2(g)→2ncl3(g); δh∘=460kj; δs∘=−275j/k
(c) n2f4(g)→2nf2(g); δh∘=85kj; δs∘=198j/k

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 09:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
Questions

Mathematics, 23.11.2019 18:31




Computers and Technology, 23.11.2019 18:31

Chemistry, 23.11.2019 18:31




Biology, 23.11.2019 18:31

Chemistry, 23.11.2019 18:31

Mathematics, 23.11.2019 18:31

History, 23.11.2019 18:31


Physics, 23.11.2019 18:31

Social Studies, 23.11.2019 18:31




Business, 23.11.2019 18:31

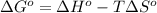
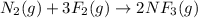
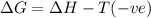
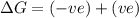
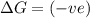 (at low Temperature)
(at low Temperature)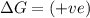 (at high Temperature)
(at high Temperature)