
Chemistry, 13.12.2019 21:31 dornauriel
Two bulbs are connected by a stopcock. the large bulb, with a volume of 6.00 l, contains nitric oxide at a pressure of 0.700 atm, 0.700 atm, and the small bulb, with a volume of 1.50 l, contains oxygen at a pressure of 2.50 atm. the temperature at the beginning and the end of the experiment is 22 ∘ c 22∘c . after the stopcock is opened, the gases mix and react. 2 no ( g ) + o 2 ( g ) → 2 no 2 ( g ) 2no(g)+o2(g)→2no2(g)
which gases are present at the end of the experiment?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
Two bulbs are connected by a stopcock. the large bulb, with a volume of 6.00 l, contains nitric oxid...
Questions

Mathematics, 13.12.2019 03:31



Spanish, 13.12.2019 03:31


Social Studies, 13.12.2019 03:31

Mathematics, 13.12.2019 03:31

History, 13.12.2019 03:31


Social Studies, 13.12.2019 03:31


History, 13.12.2019 03:31


Mathematics, 13.12.2019 03:31

History, 13.12.2019 03:31




Social Studies, 13.12.2019 03:31

Chemistry, 13.12.2019 03:31

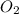 and
and 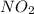
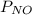 = 0
= 0

