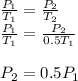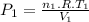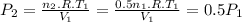Chemistry, 13.12.2019 19:31 gstinson98
Assume that you have a cylinder with a movable piston. what would happen to the gas pressure inside the cylinder if you do the following? part a: decrease the volume to one-fourth the original volume while holding the temperature constant. express your answer in terms of the variable p initial. part b: reduce the kelvin temperature to half its original value while holding the volume constant. express your answer in terms of the variable p initial .part c: reduce the amount of gas to half while keeping the volume and temperature constant. express your answer in terms of the variable p initial .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

You know the right answer?
Assume that you have a cylinder with a movable piston. what would happen to the gas pressure inside...
Questions

History, 22.01.2021 19:50


Computers and Technology, 22.01.2021 19:50

Mathematics, 22.01.2021 19:50

Chemistry, 22.01.2021 19:50


Mathematics, 22.01.2021 19:50

English, 22.01.2021 19:50




Geography, 22.01.2021 19:50



Mathematics, 22.01.2021 19:50

Physics, 22.01.2021 19:50