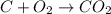Which observation illustrates the law of conservation of mass?
the c: o mass ratio of a parti...

Chemistry, 26.08.2019 16:30 xXFLUFFYXx
Which observation illustrates the law of conservation of mass?
the c: o mass ratio of a particular compound is the same, regardless of the size or source of the sample.
when 3 g of carbon reacts with 8 g of oxygen, 11 g of carbon dioxide is produced.
the c: o mass ratio of one compound is exactly double that of another compound.
burning 10 g of propane produces twice as much carbon dioxide as burning 5 g of propane.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:50
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3

Chemistry, 21.06.2019 15:00
The density of a planet is 0.69 g/cm3 (density of water is 1.0 g/cm3). which of the following planets might this be? a. mercury b. venus c. saturn d. mars
Answers: 3

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
Questions

Mathematics, 01.04.2021 06:40


Mathematics, 01.04.2021 06:40

Mathematics, 01.04.2021 06:40

Social Studies, 01.04.2021 06:40

Mathematics, 01.04.2021 06:40

Mathematics, 01.04.2021 06:40


Biology, 01.04.2021 06:40

Biology, 01.04.2021 06:40

Physics, 01.04.2021 06:40




Biology, 01.04.2021 06:40

History, 01.04.2021 06:40


Mathematics, 01.04.2021 06:40

Arts, 01.04.2021 06:40

Mathematics, 01.04.2021 06:40