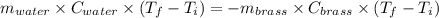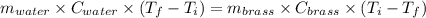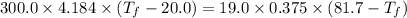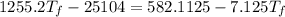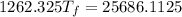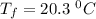Chemistry, 13.12.2019 06:31 BlueLemonWater
A19.0g sample of brass, which has a specific heat capacity of 0.375·j*g^−1°c−, is dropped into an insulated container containing 300.0g of water at 20.0°c and a constant pressure of 1atm. the initial temperature of the brass is 81.7°c.
1. assuming no heat is absorbed from or by the container, or the surroundings, calculate the equilibrium temperature of the water. be sure your answer has significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1


Chemistry, 23.06.2019 10:30
Silver is a white metal that is an excellent conductor. silver tarnishes when exposed to air and light. the density of silver is 10.49 g/cm3. the melting point is 962oc and the boiling point is 2000oc. a chemical property of silver is
Answers: 3
You know the right answer?
A19.0g sample of brass, which has a specific heat capacity of 0.375·j*g^−1°c−, is dropped into an in...
Questions


Chemistry, 05.05.2020 03:23



Mathematics, 05.05.2020 03:23

Geography, 05.05.2020 03:23

Mathematics, 05.05.2020 03:23

Mathematics, 05.05.2020 03:23






Geography, 05.05.2020 03:23

Social Studies, 05.05.2020 03:23


Mathematics, 05.05.2020 03:23

Mathematics, 05.05.2020 03:23