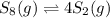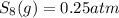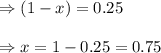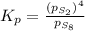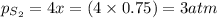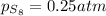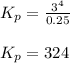1.
a sample of s8 (8) is placed in an otherwise empty rigid container at 1325 k at an in...

Chemistry, 13.12.2019 06:31 cerlos110484
1.
a sample of s8 (8) is placed in an otherwise empty rigid container at 1325 k at an initial pressure of 1.00 atm, where it
decomposes to s2 by the reaction: s8() 4 s2(2). |
at equilibrium, the partial pressure of s, is 0.25 atm. calculate k, for this reaction at 1325 k

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Questions

Mathematics, 05.02.2021 21:20




Chemistry, 05.02.2021 21:20







Mathematics, 05.02.2021 21:20

History, 05.02.2021 21:20

English, 05.02.2021 21:20

Mathematics, 05.02.2021 21:20


Mathematics, 05.02.2021 21:20

Mathematics, 05.02.2021 21:20


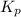 is 324
is 324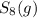 = 1.00 atm
= 1.00 atm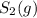 follows:
follows: