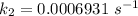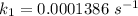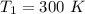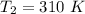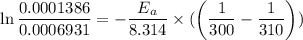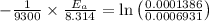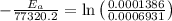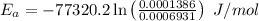Chemistry, 13.12.2019 06:31 supermimi8078
Areaction is known to exhibit 1st order kinetics. at 300k the concentration of reactant is reduced to one half of its initial value after 5000s. in contrast, at 310k the conc. is halved after 1000s. using this information to calculate: i) the rate constant for the reaction at 300kii) the time required for the reaction to be reduced to halfiii) the activation energy for the reaction

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
Areaction is known to exhibit 1st order kinetics. at 300k the concentration of reactant is reduced t...
Questions


English, 09.03.2021 22:20

Mathematics, 09.03.2021 22:20

Mathematics, 09.03.2021 22:20

Mathematics, 09.03.2021 22:20

Mathematics, 09.03.2021 22:20



Mathematics, 09.03.2021 22:20


English, 09.03.2021 22:20

Mathematics, 09.03.2021 22:20

Mathematics, 09.03.2021 22:20




Mathematics, 09.03.2021 22:20

Mathematics, 09.03.2021 22:20

Mathematics, 09.03.2021 22:20

Mathematics, 09.03.2021 22:20

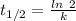
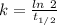
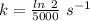
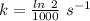
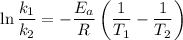

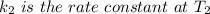
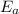 is the activation energy
is the activation energy