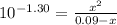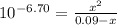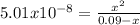Phosphorous acid, h3po3(aq), is a diprotic oxyacid that is an important compound in industry and agriculture. k pka1 k pka2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.8 m h3po3(aq) with 1.8 m koh(aq). before addition of any koh: after addition of 25.0 ml koh: after addition of 50.0 ml koh: after addition of 75.0 ml koh: after addition of 100.0 ml koh:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
Phosphorous acid, h3po3(aq), is a diprotic oxyacid that is an important compound in industry and agr...
Questions









Chemistry, 16.10.2020 02:01






Mathematics, 16.10.2020 02:01

History, 16.10.2020 02:01

History, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01

History, 16.10.2020 02:01

History, 16.10.2020 02:01