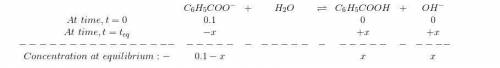Sodium benzoate (c6h5coona), the sodium salt of the weak acid benzoic acid, is used as a food preservative. a solution is prepared by dissolving 0.100 mol of sodium benzoate in enough pure water to produce 1.00 l of solution. if the pka for benzoic acid is 4.20, calculate the ph of the sodium benzoate solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Sodium benzoate (c6h5coona), the sodium salt of the weak acid benzoic acid, is used as a food preser...
Questions

Mathematics, 16.12.2021 23:40


Mathematics, 16.12.2021 23:40

Mathematics, 16.12.2021 23:40




Mathematics, 16.12.2021 23:40

Computers and Technology, 16.12.2021 23:40

History, 16.12.2021 23:40



Mathematics, 16.12.2021 23:40

Mathematics, 16.12.2021 23:40

Mathematics, 16.12.2021 23:40

Mathematics, 16.12.2021 23:40




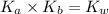
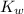 is the dissociation constant of water.
is the dissociation constant of water. ,
, 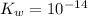
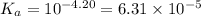
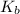 for Sodium benzoate can be calculated as:
for Sodium benzoate can be calculated as: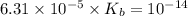
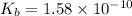
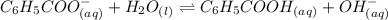
![K_{b}=\frac {\left [ C_6H_5COOH^{+} \right ]\left [ {OH}^- \right ]}{[C_6H_5COO^-]}](/tpl/images/0416/3772/af673.png)
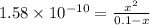
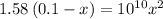
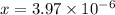
![[OH^-]=3.97\times 10^{-6}](/tpl/images/0416/3772/71435.png)
![pOH=-log[OH^-]=-log(3.97\times 10^{-6})=5.4](/tpl/images/0416/3772/9fc03.png)