Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:40
How can you increase the ability of a gas to dissolve in a liquids?
Answers: 1



Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
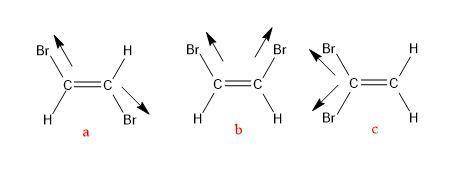
There are three different possible isomers of a dibromoethene molecule, c2h2br2c2h2br2 . one of them...
Questions

Business, 24.10.2021 01:00

Business, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

Social Studies, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00


English, 24.10.2021 01:00


Biology, 24.10.2021 01:00


Mathematics, 24.10.2021 01:00



Biology, 24.10.2021 01:00


Chemistry, 24.10.2021 01:00

Chemistry, 24.10.2021 01:00


Social Studies, 24.10.2021 01:00