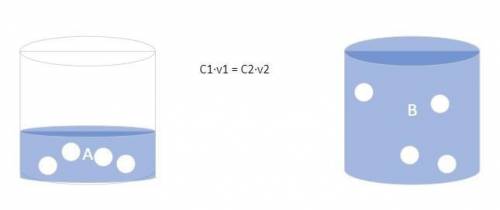
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 18.0 g of luminol to h2o creating a solution with a total volume of 75.0 ml. what is the molarity of the stock solution of luminol? before investigating the scene, the technician must dilute the luminol solution to a concentration of 5.00×10^-2 m. the diluted solution is then placed in a spray bottle for application on the desired surfaces. how many moles of luminol are present in 2.00 l of the diluted spray? what volume of the stock solution (part a) would contain the number of moles present in the diluted solution (part b)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


You know the right answer?
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 18.0 g...
Questions



Health, 05.11.2020 18:30






Computers and Technology, 05.11.2020 18:30

Computers and Technology, 05.11.2020 18:30





Arts, 05.11.2020 18:30

Biology, 05.11.2020 18:30

Mathematics, 05.11.2020 18:30