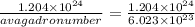Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
What would be the mass in grams of 1.204 x 1024 molecules of sulfur dioxide...
Questions


History, 20.10.2020 04:01




History, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

History, 20.10.2020 04:01



Mathematics, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01




Advanced Placement (AP), 20.10.2020 04:01


History, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

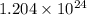 molecules =
molecules =