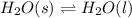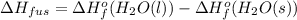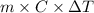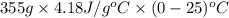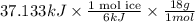Use the standard enthaplies of formation to calculate the standard change in enthaply for the melting of ice. (-291.8 kj/mol for h20 (s). use this value to calculate the mass of ice required to cool 355 ml of a beverage from the room temperature (25 degree celsius) to 0 degree celsius. assume that the specfic heat capacity and the density of the beverage are the same as those of water.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

You know the right answer?
Use the standard enthaplies of formation to calculate the standard change in enthaply for the meltin...
Questions


English, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40

Computers and Technology, 01.12.2020 18:40


Mathematics, 01.12.2020 18:40





Mathematics, 01.12.2020 18:40


Mathematics, 01.12.2020 18:40

Chemistry, 01.12.2020 18:40

History, 01.12.2020 18:40


History, 01.12.2020 18:40

Biology, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40