Chemistry, 12.12.2019 06:31 haileysolis5
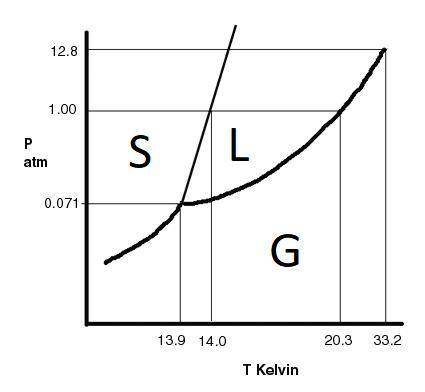
The substance hydrogen has the following properties: normal melting point: 14.0 k normal boiling point: 20.3 k triple point: 7.1×10-2 atm, 13.9 k critical point: 12.8 atm, 33.2 k at temperatures above 33.2 k and pressures above 12.8 atm, h2 is a . h2 does not exist as a liquid at pressures below atm. h2 is a at 4.84 atm and -0.2 k. h2 is a at 1.00 atm and 15.6 k. h2 is a at 7.10×10-2 atm and 34.4 k. which of the following are true?
choose all that apply
the final state of the substance is a gas.
the sample is initially a solid.
the gas initially present will solidify.
the final state of the substance is a solid.
no phase change will occur

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1


Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
The substance hydrogen has the following properties: normal melting point: 14.0 k normal boiling p...
Questions

Mathematics, 16.10.2020 19:01

Mathematics, 16.10.2020 19:01




History, 16.10.2020 19:01


Mathematics, 16.10.2020 19:01

Biology, 16.10.2020 19:01


Biology, 16.10.2020 19:01



Law, 16.10.2020 19:01


English, 16.10.2020 19:01




Mathematics, 16.10.2020 19:01