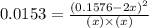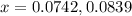Nitrogen and oxygen do not react appreciably at room temperature, as illustrated by our atmosphere. but at high temperatures, the reaction below can proceed to a measurable extent. n2(g) + o2(g) ⇔ 2 no(g) at 3000 k, the reaction above has keq = 0.0153. if 0.3152 mol of pure no is injected into an evacuated 2.0-l container and heated to 3000k, what will be the equilibrium concentration of no?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2

Chemistry, 23.06.2019 08:30
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3
You know the right answer?
Nitrogen and oxygen do not react appreciably at room temperature, as illustrated by our atmosphere....
Questions


Geography, 16.10.2019 04:30

Mathematics, 16.10.2019 04:30

Mathematics, 16.10.2019 04:30




Health, 16.10.2019 04:30












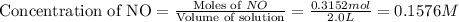
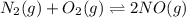
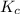 will be,
will be,![K_c=\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0414/9499/71f8f.png)