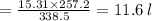Chemistry, 12.12.2019 05:31 laykaleb086
Asample of ammonia gas at 65.5°c and 524 torr has a volume of 15.31 l. what is its volume when the temperature is –15.8°c and its pressure is 524 torr? 20.2 l 11.6 l 63.5 l not possible, since the volume would have to be negative 3.69 l

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
Asample of ammonia gas at 65.5°c and 524 torr has a volume of 15.31 l. what is its volume when the t...
Questions




Biology, 12.01.2021 20:10


Mathematics, 12.01.2021 20:10

English, 12.01.2021 20:10

Mathematics, 12.01.2021 20:10

Social Studies, 12.01.2021 20:10



Mathematics, 12.01.2021 20:10

Mathematics, 12.01.2021 20:10

Biology, 12.01.2021 20:10

Mathematics, 12.01.2021 20:10

Mathematics, 12.01.2021 20:10

History, 12.01.2021 20:10

English, 12.01.2021 20:10