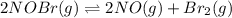Chemistry, 12.12.2019 04:31 dhurtado1195
For the following reaction, the equilibrium constant kc is 2.0 at a certain temperature. the reaction is endothermic. what do you expect to happen to the concentration of no if the temperature is doubled? 2nobr( g) ⇌ 2no( g) + br 2( g) the change in concentration of [no] will depend on the size of the vessel. the concentration of no will increase. the concentration of no will decrease. a catalyst will be needed to make a change in concentration. there will be no change in [no].

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3


Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
For the following reaction, the equilibrium constant kc is 2.0 at a certain temperature. the reactio...
Questions

Social Studies, 01.09.2019 12:00


Social Studies, 01.09.2019 12:00

Mathematics, 01.09.2019 12:00


Computers and Technology, 01.09.2019 12:00

History, 01.09.2019 12:00

Geography, 01.09.2019 12:00


Biology, 01.09.2019 12:00

Mathematics, 01.09.2019 12:00

Biology, 01.09.2019 12:00

Business, 01.09.2019 12:00



Biology, 01.09.2019 12:00


Biology, 01.09.2019 12:00

Computers and Technology, 01.09.2019 12:00

Geography, 01.09.2019 12:00