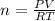Chemistry, 12.12.2019 04:31 paigebmaxwell6062
Solid aluminum reacts with aqueous h2so4 to form h2 gas and aluminum sulfate. when a sample of al is allowed to react, 415ml of gas is collected over water at 23°c at a pressure of 755mmhg. at 23°c the vapor pressure of water is 21mmhg. what is the pressure in mmhg of the dry h2 gas?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3


Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
Solid aluminum reacts with aqueous h2so4 to form h2 gas and aluminum sulfate. when a sample of al is...
Questions



Spanish, 15.01.2020 01:31


Mathematics, 15.01.2020 01:31



History, 15.01.2020 01:31

Mathematics, 15.01.2020 01:31

Mathematics, 15.01.2020 01:31

Social Studies, 15.01.2020 01:31



Biology, 15.01.2020 01:31

Mathematics, 15.01.2020 01:31

Mathematics, 15.01.2020 01:31