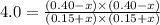Chemistry, 12.12.2019 03:31 antoniaannswiney
Acetic acid, ch3co2h, reacts with ethanol, c2h5oh, to form water and ethyl acetate, ch3co2c2h5. the equilibrium constant for this reaction with dioxane as a solvent is 4.0. what are the equilibrium concentrations for a mixture that is initially 0.15 m in ch3co2h, 0.15 m in c2h5oh, 0.40 m in ch3co2c2h5, and 0.40 m in h2o?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
Acetic acid, ch3co2h, reacts with ethanol, c2h5oh, to form water and ethyl acetate, ch3co2c2h5. the...
Questions



Mathematics, 12.02.2020 22:07



Chemistry, 12.02.2020 22:07

Mathematics, 12.02.2020 22:07












History, 12.02.2020 22:07

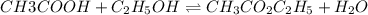
![K_c=\frac{[CH_3CO_2C_2H_5][H_2O]}{[CH_3COOH][C_2H_5OH]}](/tpl/images/0414/6294/2612b.png)