Chemistry, 12.12.2019 03:31 ayoismeisjjjjuan
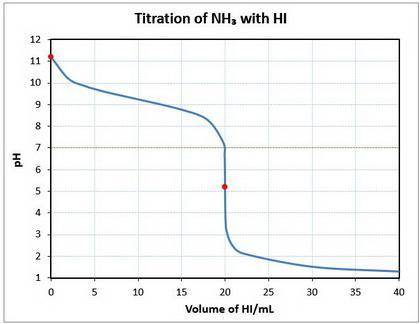
10. a 20.00 ml sample of 0.150 mol/l ammonia (nh3(aq)) is titrated to the equivalence point by 20.0 ml of a solution of 0.150 mol/l of the strong acid hydroiodic acid (hi (
a) write a balanced equation for the titration reaction.
b) what is the ph of the ammonia solution before the titration begins?
c) what is the ph at the equivalence point?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
10. a 20.00 ml sample of 0.150 mol/l ammonia (nh3(aq)) is titrated to the equivalence point by 20.0...
Questions


Mathematics, 26.09.2019 02:00



Social Studies, 26.09.2019 02:00

Geography, 26.09.2019 02:00


Business, 26.09.2019 02:00



Social Studies, 26.09.2019 02:00



History, 26.09.2019 02:00


Mathematics, 26.09.2019 02:00

World Languages, 26.09.2019 02:00

Business, 26.09.2019 02:00

Social Studies, 26.09.2019 02:00

English, 26.09.2019 02:00

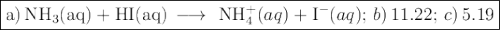

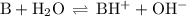
![K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.150 - x} = 1.8 \times 10^{-5}](/tpl/images/0414/6924/2256c.png)
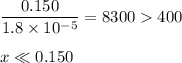
![\dfrac{x^{2}}{0.150} = 1.8 \times 10^{-5}\\\\x^{2} = 0.150 \times 1.8 \times 10^{-5}\\x^{2} = 2.7 \times 10^{-6}\\x = \sqrt{2.7 \times 10^{-6}}\\x = \text{[OH]}^{-} = 1.64 \times 10^{-3} \text{ mol/L}](/tpl/images/0414/6924/0b94c.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(1.64 \times 10^{-3}) = 2.78\\\\\text{pH} = 14.00 - \text{pOH} = 14.00 - 2.78 = \mathbf{11.22}\\\\\text{The pH of the solution at equilibrium is } \large \boxed{\mathbf{11.22}}](/tpl/images/0414/6924/f4986.png)
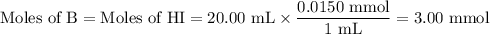
![\rm [BH^{+}] = \dfrac{\text{3.00 mmol}}{\text{40.00 mL}} = \text{0.0750 mol/L}](/tpl/images/0414/6924/607b2.png)
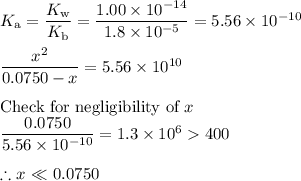
![\dfrac{x^{2}}{0.0750} = 5.56 \times 10^{-10}\\\\x^{2} = 0.0750 \times 5.56 \times 10^{-10}\\x^{2} = 4.17 \times 10^{-11}\\x = \sqrt{4.17 \times 10^{-11}}\\\rm [H_{3}O^{+}] =x = 6.46 \times 10^{-6}\, mol \cdot L^{-1}](/tpl/images/0414/6924/dc4d9.png)
![\text{pH} = -\log{\rm[H_{3}O^{+}]} = -\log{6.46 \times 10^{-6}} = \large \boxed{\mathbf{5.19}}](/tpl/images/0414/6924/b2d28.png)